In vitro insights play a vital role in navigating the complex landscape of toxicology testing services. This article delves into the importance of in vitro testing, highlighting its distinct advantages and wide-ranging applications.
Exploring key considerations for selecting toxicology testing services, it also provides a glimpse into future trends in this field.
By adopting a professional and technical approach, this article aims to inform and guide professionals seeking to navigate the intricacies of toxicology testing services.
The Importance of In Vitro Testing
Source: rockstepsolutions.com
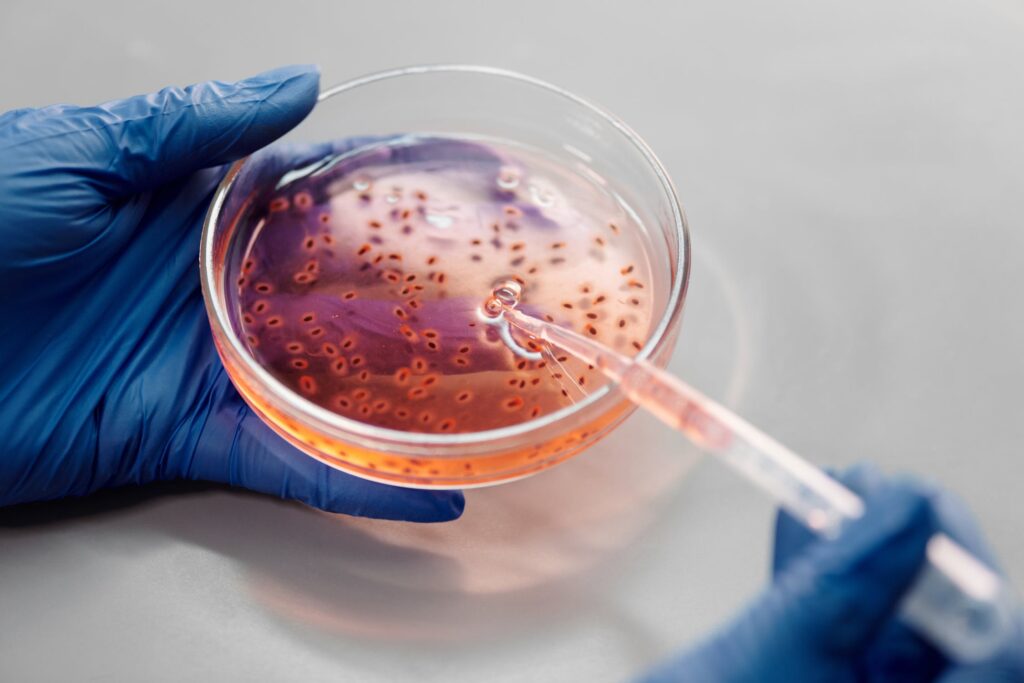
In vitro testing plays a crucial role in assessing the potential toxic effects of substances, providing accurate and reliable data for regulatory and safety evaluations.
Traditionally, the role of animals in toxicology testing has been significant, with animal models used to predict human responses to various compounds. However, there has been a growing recognition of the limitations and ethical concerns associated with animal testing.
As a result, regulatory agencies have increasingly accepted and encouraged the use of in vitro testing methods as alternatives. In vitro testing offers several advantages, including the ability to study human cells directly, allowing for more accurate predictions of human responses to substances.
Furthermore, in vitro testing provides faster results, lower costs, and reduced reliance on animal models, thus promoting the refinement and reduction of animal testing in toxicology assessments.
The regulatory acceptance of in vitro testing as a reliable and valid method has driven its widespread adoption in the field of toxicology.
Advantages of in Vitro Insights

Incorporating in vitro insights into toxicology testing services offers numerous advantages in terms of accuracy, efficiency, and ethical considerations. In vitro research, which involves studying biological processes outside of living organisms, allows for controlled experiments using cellular models. This approach provides researchers with a better understanding of the effects of chemicals on human health, without the need for animal testing.
By utilizing cellular models, scientists can simulate specific organs or tissues, enabling them to assess toxicity more accurately. In vitro testing also offers increased efficiency, as it allows for high-throughput screening of chemicals, reducing the time and cost associated with traditional animal testing.
Furthermore, this method aligns with ethical considerations, as it minimizes the use of animals in research while still providing valuable insights into toxicology.
Applications of in Vitro Testing

Source: criver.com
By leveraging the advantages of in vitro testing, researchers can apply this approach to a wide range of toxicology applications, providing valuable insights into the effects of chemicals on human health.
In vitro testing offers an alternative method to traditional animal testing, allowing for more accurate and reliable results. This method involves conducting experiments in controlled laboratory conditions using human cells or tissues, mimicking the biological processes that occur in the human body.
The use of in vitro testing has gained regulatory acceptance in recent years, with organizations such as the Organization for Economic Co-operation and Development (OECD) endorsing its use for certain toxicological evaluations. This acceptance highlights the potential of in vitro testing to replace or reduce the need for animal testing in regulatory frameworks, making it an important tool in toxicology research and ensuring the safety of chemicals on human health.
Key Considerations for Selecting Toxicology Testing Services

Source: lek.com
Researchers must carefully consider several key factors when selecting toxicology testing services, ensuring that the chosen provider can effectively meet their specific research needs.
One of the primary considerations is the availability and expertise in a wide range of toxicology testing methods. Different methods, such as in vitro assays, animal models, and computational modeling, may be necessary depending on the research objectives.
Another crucial factor is compliance with regulatory requirements. The chosen service provider should have a thorough understanding of the specific regulations governing toxicology testing, such as Good Laboratory Practice (GLP) guidelines. This ensures that the research is conducted in a manner that meets the necessary standards and can be accepted by regulatory authorities.
Additionally, researchers should assess the provider’s track record, reputation, and experience in conducting toxicology testing to ensure reliability and accuracy of results.
Future Trends in In Vitro Toxicology Testing

Source: nextmsc.com
The future of in vitro toxicology testing holds promising advancements and possibilities. As the field continues to evolve, future innovations and emerging technologies are expected to enhance the accuracy, efficiency, and predictive power of in vitro testing methods.
One key area of development is the integration of high-throughput screening (HTS) platforms with advanced data analysis tools, such as artificial intelligence and machine learning algorithms. These technologies allow for the rapid screening of large numbers of compounds and the identification of potential toxicological effects.
Additionally, the use of organ-on-a-chip models and 3D cell culture systems is expected to become more prevalent in the future, enabling researchers to better mimic human physiology and improve the relevance and reliability of in vitro toxicology testing.
FAQ’s
What Are the Potential Risks and Limitations Associated With in Vitro Testing Methods?
In vitro testing methods have potential risks and limitations. Risks include the inability to accurately replicate the complex interactions of a whole organism, while limitations involve the challenges of extrapolating results to predict effects in vivo.
How Does in Vitro Testing Compare to Traditional Animal Testing in Terms of Accuracy and Reliability?
In vitro testing offers a promising alternative to traditional animal testing, with advantages in terms of accuracy and reliability. It allows for controlled experiments in a controlled environment, reducing variability and providing more precise and informative results.
Are There Any Regulatory Requirements or Guidelines That Govern the Use of in Vitro Testing for Toxicology Assessments?
Regulatory requirements and guidelines are in place to govern the use of in vitro testing for toxicology assessments. These guidelines ensure that the testing is conducted accurately and reliably, providing valuable data for assessing the safety of chemicals and products.
What Are the Main Factors to Consider When Selecting a Toxicology Testing Service Provider for in Vitro Testing?
When selecting a toxicology testing service provider for in vitro testing, several factors should be considered. These include expertise in the field, adherence to regulatory guidelines, availability of advanced technologies, and track record of delivering accurate and reliable results.
Are There Any Emerging Technologies or Advancements in In Vitro Toxicology Testing That Could Significantly Impact the Field in the Near Future?
Emerging technologies and advancements in in vitro toxicology testing services have the potential to significantly impact the field in the near future. These innovations can enhance accuracy, efficiency, and predictive capabilities, leading to improved safety assessments and faster drug development processes.



